GcMAF (Gc Protein derived Macrophage Activating Factor)
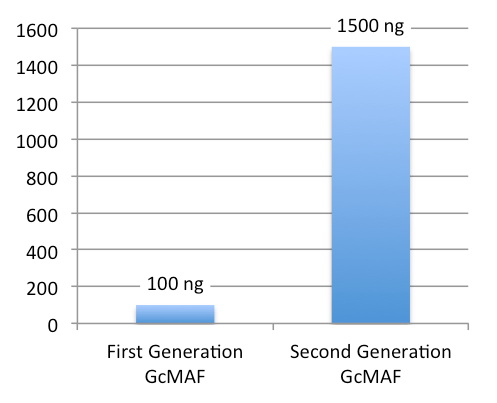
Comparison of 1st and 2nd Generation GcMAF
Comparison of 1st and 2nd Generation GcMAF
First Generation GcMAF

- Developed by Dr Yamamoto in 1991
- Low concentration (100 ng/0.25 ml, 1 dose)
- Low stability at room temperature and when refrigerated
- Gc protein isolated from serum using 25-(OH) Vitamin D3 Affinity Column, making the GcMAF unstable
- Sterilized using 0.22 micron sterile filtration system
- Tokushima University conducts experiments with 1st Generation and 2nd Generation GcMAF
Goleic GcMAF, GcMAF with oleic acid, is simply first generation GcMAF with some added components but still having the low concentration of the original first generation GcMAF developed over 20 years ago. Due to limited testing and clinical use, the efficacy of Goleic GcMAF is unknown.
Second Generation GcMAF



- 2nd Generation High Dose GcMAF Developed by the University of Tokushima and Saisei Mirai in 2011
- New patented production process
- High concentration (1500 ng/0.5 ml, 1 dose)
- Very high macrophage activating activity
- Much higher stability even at room temperature *
- Demonstrated in vitro to increase the rate of maturation of Dendritic Cells (DCs).
- Sterilized using 0.22 micron sterile filtration system
* Second Generation GcMAF is best stored refrigerated and will stay fully active for over 1 year. Stability tests indicate that 2nd Generation GcMAF is very temperature stable and retains maximum activity even after 4 weeks at room temperature and 1 week at 40 °C (104 °F).
| 1991 | Dr Yamamoto developed GcMAF |
| 1992 | Dr Yamamoto visited Dr Hori at Tokushima University GcMAF research started at Tokushima University |
| 1998 | Dr Uto joined Dr Hori’s GcMAF research team |
| 2002 | First research papers published on GcMAF in the journals Biotherapy and Comparative Biochemistry & Physiology |
| 2010 | Tokushima University began collaborating with Saisei Mirai to develop Second Generation High Dose GcMAF |
| 2011 | Second Generation GcMAF produced in our Cell Processing Center (CPC) for patients. Start of clinical use. |
| 2013 | Two research papers published in Anticancer Research by Saisei Mirai & Tokushima University |
| 2013 | Over 1000 patients treated with Saisei Mirai GcMAF |
| 2014 | New research paper published in Anticancer Research by Saisei Mirai & Tokushima University |
If you wish to purchase GcMAF therapy or make an enquiry, please contact us with details of your disease, current treatment and the quantities of Gc-MAF you require.
