GcMAF (Gc Protein derived Macrophage Activating Factor)
Research and references GcMAF
Tests of Second Generation GcMAF
Experiment report
14-JUN-2012 Stability of GcMAF in Serum (PDF)
H Mukai, Y Uto. Department of Biological Science and Technology, The University of Tokushima.
- This experiment was conducted at the University of Tokushima in Japan, using our 2nd generation GcMAF demonstrating macrophage phagocytic activity after short-term and long-term storage conditions. The results show that 2nd generation GcMAF in serum is stable for 1 year at 4 °C, for 14 days at room temperature (around 20 °C), and for 7 days at 40 °C.
- Please note, this research only applies to second generation GcMAF produced with our Patent Pending production process. GcMAF produced using the old method, using vitamin D affinity chromatography, does not have the same stability as 2nd generation GcMAF because it is susceptible to much more rapid oxidation. This difference in stability is due to the different production process, not due to the GcMAF itself.
Macrophage phagocytic activity assay of Second Generation GcMAF
Macrophage phagocytic activity assay using mouse macrophages
Second generation GcMAF is tested for macrophage phagocytic activity using mouse macrophages and sheep red blood cells at the University of Tokushima. The red blood cells are opsonized which marks them for ingestion and destruction by activated macrophages, seen as purple areas in the clear cells. From this we calculate the Phagocytosis (ingestion) Index (PI).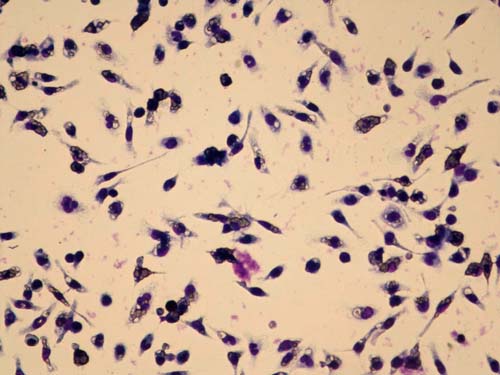
Preliminary results of in vivo experiments in cancer-bearing mice
In vivo cancer-bearing mouse experiments have been done with our 2nd generation GcMAF. Cancer-bearing mice were treated with 2nd generation GcMAF, purified GcMAF produced using Dr Yamamoto’s method and saline control. Second generation GcMAF was much more effective than purified GcMAF in these experiments. (Unpublished results, University of Tokushima. Research paper expected to be published in 2013).
Published research papers on GcMAF by our collaborators
We collaborate with GcMAF researchers at the University of Tokushima, Japan in the development of second generation GcMAF. Below are published research papers on GcMAF in peer-reviewed scientific journals authored by University of Tokushima researchers over the last decade. Our research on GcMAF is ongoing and more papers are being prepared for publication in the future.
2010 Vitamin D Binding Protein-Macrophage Activating Factor Inhibits HCC in SCID Mice (PDF)
K Nonaka, S Onizuka, H Ishibashi, Y Uto, H Hori, T Nakayama, N Matsuura, T Kanematsu, H Fujioka.
- Background. A high incidence of recurrence after treatment is the most serious problem in hepatocellular carcinoma (HCC). Therefore, a new strategy for the treatment of the disease is needed. The aim of the present study was to investigate whether vitamin D binding protein-macrophage activating factor (DBPmaf) is able to inhibit the growth of HCC. Conclusion. DBP-maf has at least two novel functions, namely, an anti-angiogenic activity and tumor killing activity through the activation of macrophages. DBP-maf may therefore represent a new strategy for the treatment of HCC.
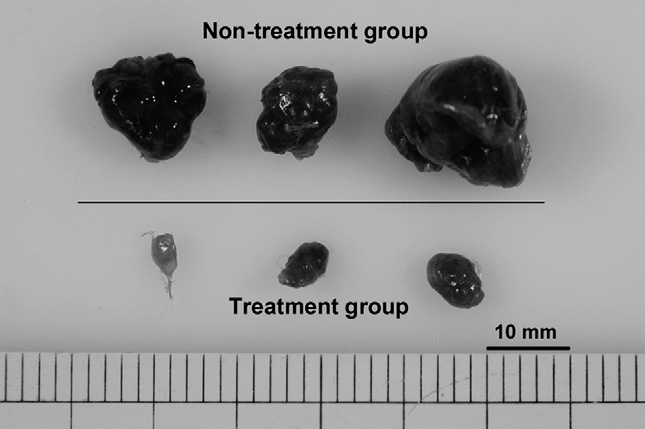
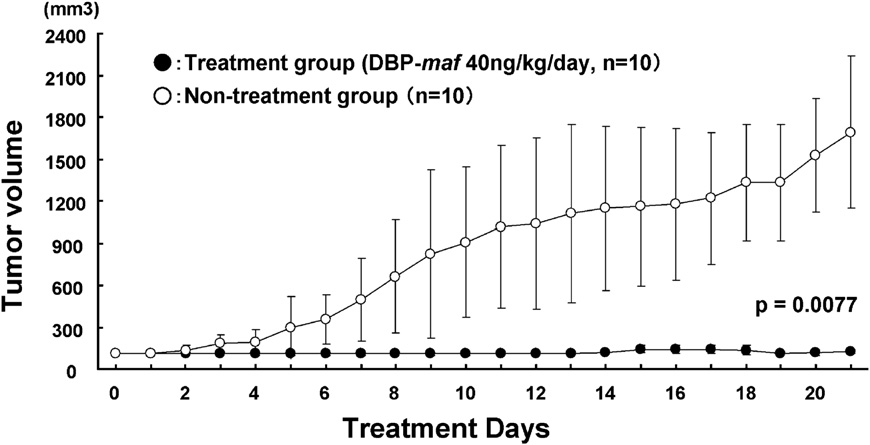
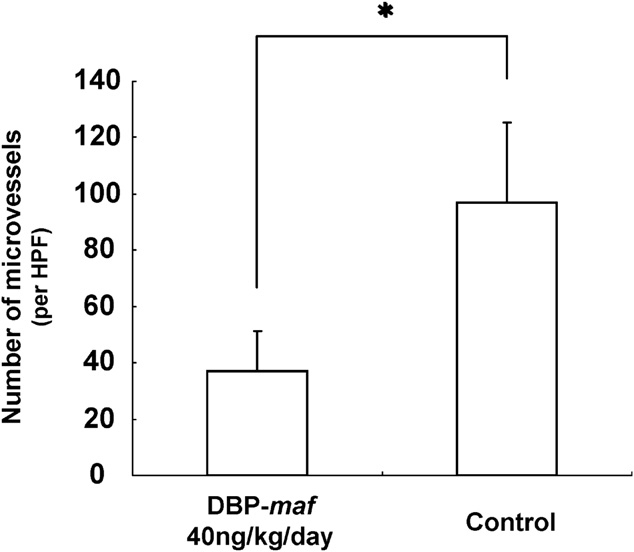
- Author’s comments:
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. The therapeutic options for HCC are limited and frequent recurrences develop even after curative treatments. Solid tumors, including HCC, must establish an adequate vascular network to acquire nutrition. Tumor angiogenesis is one of the fundamental requirements for tumor growth and proliferation.
We have previously reported that macrophage activating factor derived from vitamin D binding protein (DBP) is a novel angiogenesis inhibitor found in the medium of the human pancreatic cancer cell line, BxPC-3. The present in vivo study demonstrated that DBPmaf can reduce tumor progression of xenografted HCC cells in SCID mice. The in vitro study also showed that DBP-maf has at least two biologic functions; antiangiogenic activity and enhancement of macrophage activation.
Anti-angiogenesis is considered to be one of the most effective methods for inducing tumor regression or dormancy. Reports by others support our results, in which DBP-maf acts as an anti-angiogenic agent and inhibits the growth of HCC in mice.
Hepatocellular carcinoma has been reported to show increased MVD, as well as increased VEGF levels in the serum or tumor tissue. Bevacizumab (Avastin; a humanized murine anti-VEGF monoclonal antibody) is clinically used to treat colorectal cancer. Several studies have explored the use of bevacizumab as a single agent or in combination with cytotoxic or molecularly targeted agents in patients with advanced HCC. DBP-maf has been shown to exert its antiangiogenic effect through the inhibition of the VEGF signaling cascade, in a similar manner as bevacizumab. All of these results indicate that DBP-maf is a promising angiogenesis inhibitor, although further exploration of its mode of action is required.
Our present studies demonstrated that DBP-maf induced the massive infiltration of macrophages into the transplanted HCC cells and also activated macrophages as antigen presentation cells against tumor antigens. These macrophages thus not only acted as innate immune cells, but also played the same role as dendritic cells.
We observed no antiproliferative effect on HCC cells by DBP-maf itself, thus suggesting that DBP-maf synergistically functioned by inhibiting angiogenesis and killing tumor cells as a result of the activated macrophages.
We have shown for the first time that the treatment of SCID mice bearing subcutaneous HepG2 with DBP-maf resulted in tumor growth suppression.
[PubMed] [PDF version]
2005 Gc Protein (Vitamin D-binding Protein): Gc Genotyping and GcMAF Precursor Activity (PDF)
H Nagasawa, Y Uto, H Sasaki, N Okamura, A Murakami, S Kubo, KL Kirk, H Hori.
- Abstract. The Gc protein (human group-specific component (Gc), a vitamin D-binding protein or Gc globulin), has important physiological functions that include involvement in vitamin D transport and storage, scavenging of extracellular G-actin, enhancement of the chemotactic activity of C5a for neutrophils in inflammation and macrophage activation (mediated by a GalNAc-modified Gc protein (GcMAF)). In this review, the structure and function of the Gc protein is focused on especially with regard to Gc genotyping and GcMAF precursor activity. A discussion of the research strategy “GcMAF as a target for drug discovery” is included, based on our own research.
[PubMed] [PDF version]
2004 Association of the Macrophage Activating Factor (MAF) Precursor Activity with Polymorphism in Vitamin D-binding Protein (PDF)
H Nagasawa, H Sasaki, Y Uto, S Kubo, H Hori.
- Abstract. Background. Serum vitamin D-binding protein (Gc protein or DBP) is a highly expressed polymorphic protein, which is a precursor of the inflammation-primed macrophage activating factor, GcMAF, by a cascade of carbohydrate processing reactions. In order to elucidate the relationship between Gc polymorphism and GcMAF precursor activity, we estimated the phagocytic ability of three homotypes of Gc protein, Gc1F-1F, Gc1S-1S and Gc2-2, through processing of their carbohydrate moiety. Methods. We performed Gc typing of human serum samples by isoelectric focusing (IEF). Gc protein from human serum was purified by affinity chromatography with 25-hydroxyvitamin D3-sepharose. A phagocytosis assay of Gc proteins, modified using beta-glycosidase and sialidase, was carried out. Results. The Gc1F-1F phenotype was revealed to possess Galbeta1-4GalNAc linkage by the analysis of GcMAF precursor activity using beta1-4 linkage-specific galactosidase from jack bean. The GcMAF precursor activity of the Gc1F-1F phenotype was highest among three Gc homotypes. Conclusion. The Gc polymorphism and carbohydrate diversity of Gc protein are significant for its pleiotropic effects.
[PubMed] [PDF version]
2003 Characterization of human Gc protein-derived macrophage activation factor (GcMAF) and its functional role in macrophage tumoricidal activity (PDF)
S Mohamad, H Hori, H Nagasawa, K Usui, Y Uto.
- Introduction. Macrophages are essential for host defense and play an important role in orchestrating immune response of the host against threat signals. Macrophages are also known to have a critical role in antitumor immunity, can infiltrate into tumor, and are found in most tumor sites. Meanwhile, Gc protein (also known as vitamin D3-binding protein) is a serum protein with multifunctional properties and has been reported as a precursor for macrophage activation factor. Gc protein can be converted by an inducible B-galactosidase of B cells and neuraminidase of T cells to a potent macrophage activating factor (GcMAF), a protein with N-acetylgalactosamine (GalNAc) as the remaining sugar moiety. Activated macrophages express tumoricidal activity by ingestion of tumor cells and release of reactive oxygen species (ROS) and reactive nitrogen species (RNS), or both. We reported that in situ modification of Gc protein with B-galactosidase and neuraminidase increased the release of superoxide in thioglycolate-elucidated mouse peritoneal macrophage. Yamamoto et al. reported the possibility of using GcMAF as an immunomodulator for cancer treatment, so it is important to provide an assay for GcMAF. Kanan et al. reported the quantitative analysis of GcMAF from human serum. However, the sugar moiety of GcMAF has never been qualitatively studied. Here we present the study of qualitative analysis of GcMAF from purified human serum as well as its influence on the macrophage activity.[PubMed] [PDF version]
2003 Gc Protein-derived Macrophage Activating Factor (GcMAF): Isoelectric Focusing Pattern and Tumoricidal Activity (PDF)
S Mohamad, H Nagasawa, H Sasaki, Y Uto, Y Nakagawa, K Kawashima, H Hori.
- Background. Gc protein is the precursor for Gc protein derived macrophage activating factor (GcMAF), with three phenotypes: Gc1f, Gc1s and Gc2, based on its elecrophoretic mobility. The difference in elecrophoretic mobility is because of the difference in its posttranslational sugar moiety composition. Materials and Methods. We compared the difference between Gc protein and GcMAF elecrophoretic mobility using the isoelectric focusing (IEF) method. The tumoricidal activity of GcMAF treated macrophage was evaluated after coculture with L-929 cell. The tumoricidal mechanism was investigated using TNF bioassay and nitric oxide (NO) release. Results. The difference in Gc protein and GcMAF elecrophoretic mobility was detected. The tumoricidal activity of GcMAF-treated macrophage was detected, but no release of TNF and NO was detected. Conclusion. The difference of isoelectric focusing mobility in Gc protein and GcMAF would be useful to develop a GcMAF detection method. GcMAF increased macrophage tumoricidal activity but TNF and NO release were not involved in the mechanism.
[PubMed] [PDF version]
2002 Tumor cell alpha-N-acetylgalactosaminidase activity and its involvement in GcMAF-related macrophage activation (PDF)
S Mohamad, H Nagasawa, Y Uto, H Hori.
- Abstract. Alpha-N-acetyl galactosaminidase (alpha-NaGalase) has been reported to accumulate in serum of cancer patients and be responsible for deglycosylation of Gc protein, which is a precursor of GcMAF-mediated macrophage activation cascade, finally leading to immunosuppression in advanced cancer patients. We studied the biochemical characterization of alpha-NaGalase from several human tumor cell lines. We also examined its effect on the potency of GcMAF to activate mouse peritoneal macrophage to produce superoxide in GcMAF-mediated macrophage activation cascade. The specific activity of alpha-NaGalases from human colon tumor cell line HCT116, human hepatoma cell line HepG2, and normal human liver cells (Chang liver cell line) were evaluated using two types of substrates; GalNAc-alpha-PNP (exo-type substrate) and Gal-beta-GalNAc-alpha-PNP (endo-type substrate). Tumor-derived alpha-NaGalase having higher activity than normal alpha-NaGalase, had higher substrate specificity to the exo-type substrate than to the endo-type substrate, and still maintained its activity at pH 7. GcMAF enhance superoxide production in mouse macrophage, and pre-treatment of GcMAF with tumor cell lysate reduce the activity. We conclude that tumor-derived alpha-NaGalase is different in biochemical characterization compared to normal alpha-NaGalase from normal Chang liver cells. In addition, tumor cell-derived alpha-NaGalase decreases the potency of GcMAF on macrophage activation.
[PubMed] [PDF version]
2002 Preparation of Gc Protein-derived Macrophage Activating Factor (GcMAF) and its Structural Characterization and Biological Activities (PDF)
S Mohamad, H Nagasawa, Y Uto, H Hori.
- Abstract. Background. Gc protein has been reported to be a precursor of Gc protein-derived macrophage activation factor (GcMAF) in the inflammation-primed macrophage activation cascade. An inducible beta-galactosidase of B cells and neuraminidase of T cells convert Gc protein to GcMAF. Results. We successfully purified Gc protein from human serum. GcMAF was detected by lectin blotting and showed a high biological activity. Conclusion. Our results support the importance of the terminal N-acetylgalactosamine moiety in the GcMAF-mediated macrophage activation cascade, and the existence of constitutive GcMAF in human serum. These preliminary data are important for designing small molecular GcMAF mimics.
[PubMed] [PDF version]
Other published research papers on Gc-MAF
2011 Polymorphism in vitamin D-binding protein as a genetic risk factor in the pathogenesis of endometriosis (PDF)
K Faserl, G Golderer, L Kremser, H Lindner, B Sarg, L Wildt, B Seeber.
- Abstract. Previous studies have implicated a deficiency in the inflammatory response in women who develop endometriosis. The specific immunological deficits have not been completely elucidated. Objective. Our objective was to identify differences in protein expression in serum that might shed light on the pathophysiology of endometriosis. Method. This cross-sectional study of women undergoing laparoscopy between 2003 and 2005 took place at a university medical center. Patients included consenting women age 18–49 yr undergoing surgery for pain and/or infertility or elective tubal ligation. Blood was collected preoperatively. Main Outcome Measure. Proteomic analysis of serum was done using two-dimensional difference gel electrophoresis. Results. We found 25 protein spots with a significant difference in abundance between women with endometriosis and controls, including acute-phase proteins and complement components. The abundance of vitamin D-binding protein was higher in all endometriosis pools by a factor of approximately 3 compared with the control pool. Analysis of specific allele products using nano-scalec liquid chromatography-electrospray ionization-mass spectrometry indicated that it was the GC*2 allele product that was in greater concentration in serum pools, as well as in single validation samples, in women with endometriosis. In contrast to the GC*1 allele product, which is readily converted to a potent macrophage factor (Gc protein-derived macrophage activating factor), the GC*2 allele product undergoes practically no such conversion. Conclusions. We speculate that the inability to sufficiently activate macrophages’ phagocytotic function in those carrying the GC*2 polymorphism (more prevalent in endometriosis) may allow endometriotic tissues to implant in the peritoneal cavity. Future studies evaluating specific vitamin D-binding protein polymorphisms as a risk factor for endometriosis in larger populations of women are warranted.
- Author’s comments:
Endometriosis is a common disease of reproductive age women. It affects about 10% of all women and upwards of 40% of women with infertility. Women with endometriosis may have an impairment of their immune systems and inflammatory responses, allowing for the development of endometriotic lesions.
Our most impressive discovery is the differential abundance of a specific allele product of DBP, GC*2. The expression of the GC*2 allele product was 3-fold higher in all endometriosis pools compared with the control pool.
Besides acting as a transporter for vitamin D metabolites, DBP is the most potent activator for macrophages in the form of Gc protein-derived macrophage-activating factor (GcMAF). The GC*1 allele products are glycosylated at a total rate of 10–30%, whereas the GC*2 allele product is glycosylated at a rate of 1–5%. Thus, the form of DBP encoded by the GC*1 allele is much more readily converted to GcMAF, whereas that encoded by GC*2 is converted hardly at all.
Those with only GC*2 allele products, disproportionally represented in the endometriosis group, have a much reduced capability to convert DBP to GcMAF, the critical macrophage activator.
Based on the findings of our study, we speculate that the immune defect may lie, at least in part, in the inability to sufficiently activate macrophages’ phagocytotic function in those carrying the GC*2 allele of DBP.
The activation of macrophages via targeted immunotherapy in affected women might form the basis of a novel treatment strategy for endometriosis.
[PubMed] [HTML version] [PDF version]
