Oral intake of degalactosylated
whey protein increases peripheral
blood telomere length in young
and aged mice
The Effects of Dietary Intervention and Macrophage-Activating
Factor Supplementation on Cognitive Function in Elderly Users of Outpatient Rehabilitation
Degalactosylated Whey Protein Suppresses Inflammatory Responses Induced by Lipopolysaccharide in Mice
The Effect of MAF Capsules and M Capsules on Lymphopenia and Clinical Outcomes in Non-Critical Hospitalized COVID-19 Patients
Adjunctive use of oral MAF is associated with no disease progression or mortality in hospitalized patients with COVID-19 pneumonia: The single-arm COral-MAF1 prospective trial (Italy)
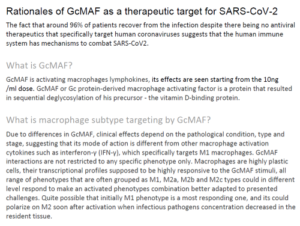
The hypothesis: Based on the aforementioned findings and on documented analogies between SARS-CoV-2 and HIV, we hypothesized that the reduced conversion activity of the Gc protein (human group-specific component (Gc)) into the macrophage activating factor (MAF) could have a key role in the dysregulate immune response induced by SARS-CoV-2, just like for HIV infected patients. If this hypothesis is correct, it might help to set a valid strategy of immunotherapy also based on an off-label use of GcMAF in critically ill COVID-19 patients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7513798/
…

Research paper
2016 Case Report: GcMAF Treatment in a Patient with Multiple Sclerosis (PDF)
ANTICANCER RESEARCH 36: 3771-3774 (2016)

About the Sonodynamic and Photodynamic Therapy (3 papers)
Conference presentation

Conference presentation - 9th International Congress for Medical Laser Applications, Germany
29-JUN-2014
Indications for GcMAF for immunotherapy of cancers and chronic viral and bacterial infections (PDF) Dr. Toshio Inui

Conference presentation - The 17th Annual Meeting of The Society of Biotherapeutic Approaches, Fukuoka University
7-DEC-2013
Case Report: A Breast Cancer Patient Treated with GcMAF, Sonodynamic Therapy and Hormone Therapy (PDF), PPT Dr. Toshio Inui
Our research group

- Professor Hitoshi Hori, Institute of Technology and Science, The University of Tokushima, Tokushima, Japan.
- Associate professor, Yoshihiro Uto, Institute of Technology and Science, The University of Tokushima, Tokushima, Japan.
- Professor Norihiro Sakamoto, National University Hospital, Kobe University School of Medicine, Kobe, Japan.
- Professor Yoshinori Marunaka, Kyoto Prefectural University of Medicine, Kyoto, Japan.
- Professor Yoshito Nishikata, Faculty of Science, Konan University, Kobe, Japan.
- Kentaro Kubo, PhD., Saisei Mirai Cell Processing Center, Osaka, Japan.