There are different generations in GcMAF therapy. The first generation GcMAF was developed over 20 years ago. Due to limited testing and clinical use, the efficacy is unknown.
We developed 2nd Generation and 3rd Generation GcMAF with a new patented production process, which makes our GcMAF very unique.
Please note that our 2nd Generation GcMAF is not be a colourless liquid, because it is produced from serum. Any GcMAF which is a colourless liquid is not 2nd Generation GcMAF produced by Saisei Mirai and may potentially be a fake product.
Our 3rd Generation GcMAF products are classified as a food product in Japan, and they are available in the form of capsules, powders, sprays (discontinued) and lollies (lozenges).
Our 3rd Generation GcMAF products are not produced from human serum, but from cow’s milk.
Oral Colostrum MAF Capsules is produced from bovine colostrum. Colostrum is very similar to serum – very rich in protein, IgG, IgA and IgM. MAF is now available in capsules, powders, sprays (discontinued) and lollies (lozenges). MAF Spray (discontinued) and M-Lollies are produced from cheese extract. All can be administered orally and topically.
Comparison of 1st and 2nd Generation GcMAF
First Generation GcMAF
- Developed by Dr Yamamoto in 1991
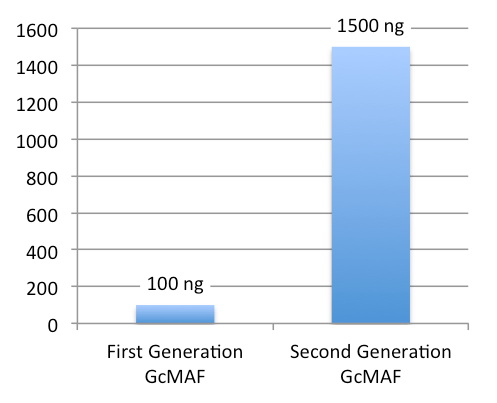
- Low concentration (100 ng/0.25 ml, 1 dose)
- Low stability at room temperature and when refrigerated
- Gc protein isolated from serum using 25-(OH) Vitamin D3 Affinity Column, making the GcMAF unstable
- Sterilized using 0.22 micron sterile filtration system
- Tokushima University conducts experiments with 1st Generation and 2nd Generation GcMAF
Second Generation GcMAF
- 2nd Generation High Dose GcMAF Developed by the University of Tokushima and Saisei Mirai in 2011
- New patented production process
- High concentration (1500 ng/0.5 ml, 1 dose)
- Very high macrophage activating activity
- Much higher stability even at room temperature *
- Demonstrated in vitro to increase the rate of maturation of Dendritic Cells (DCs).
- Sterilized using 0.22 micron sterile filtration system
Third Generation GcMAF (Capsules, Sprays and Lollies)
- Developed by Saisei Mirai and Tokushima University in 2014
- Registered as a food product in Japan
Oral Colostrum MAF Capsules
- MAF produced from bovine colostrum
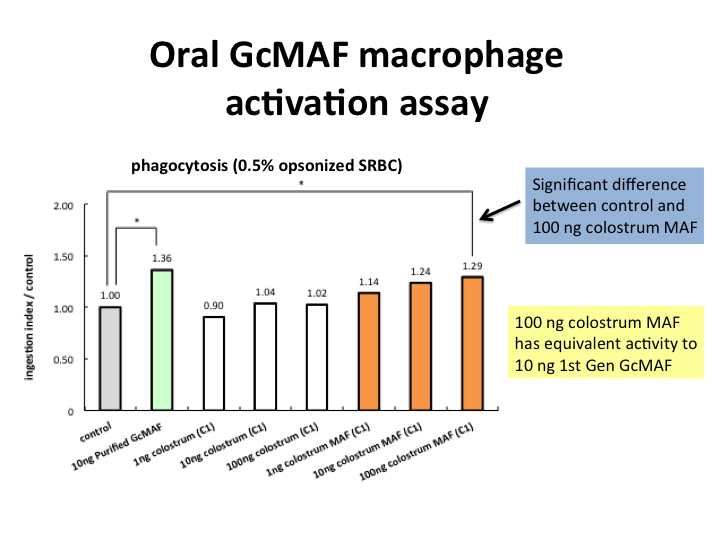
- 1 mg capsule has equivalent activity to 100ng purified GcMAF
- Enteric acid-resistant capsule for oral administration, powder for sublingual
- Target Payer‘s Patches/Gut
MAF Spray (discontinued)
- MAF produced from cheese extract
- 1 dose (2 sprays) has equivalent activity to 100 ng purified GcMAF
- MAF Spray is designed for oral and skin application.
M-Lollies
- MAF produced from cheese extract
- 1 lolly has equivalent activity to 100 ng purified GcMAF
- In the form of candies (lozenges) for easy, every day consumption.