New GcMAF
NEW GcMAF is produced using a patented process developed in Japan by Saisei Mirai, in collaboration with researchers from the University of Tokushima, who have been conducting GcMAF research for over 20 years. Manufactured in our sterile cell processing facility, this advanced formulation is 10–15 times more concentrated than conventional GcMAF and offers significantly enhanced stability and bioactivity.
Clinically, this higher-concentration GcMAF has demonstrated a favorable safety profile, with the vast majority of patients experiencing no adverse effects. In rare cases (approximately 1 in 100), mild and transient symptoms such as low-grade fever or eczema may occur. Its improved resistance to oxidation contributes to greater stability, making NEW GcMAF a reliable and effective option for immune support.
First-Generation GcMAF vs. Saisei Mirai NEW GcMAF (formerly 2nd Generation)
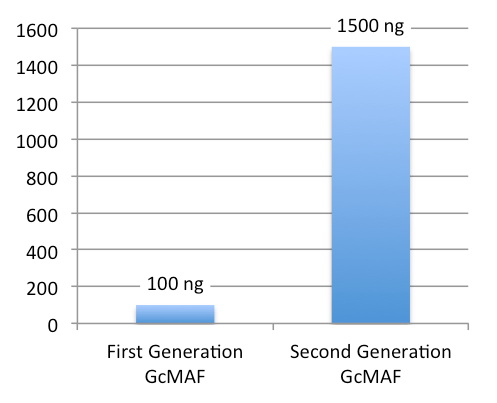
New GcMAF – Concentration and Clinical Use
Each 0.5 mL vial of New GcMAF contains approximately 1,500 ng of GcMAF.
GcMAF is a naturally derived immunotherapy product. As with other immune components such as lymphocytes and natural killer (NK) cells, the concentration of GcMAF may vary slightly due to individual differences in serum composition.
At Saisei Mirai, New GcMAF is produced under strict aseptic conditions in a certified cell processing facility. All products undergo sterile filtration to ensure safety and purity.
Since April 2011, New GcMAF (formerly known as 2nd Generation GcMAF) has been safely administered to hundreds of patients in our clinics across Japan. Clinical application methods have included intramuscular (IM), subcutaneous (SC), and intratumoral (IT) injections.
Our specialized sterile Cell Processing Center (CPC) and team of highly skilled laboratory staff






How is Saisei Mirai’s New GcMAF Made?
Saisei Mirai’s New GcMAF is produced in our own sterile, certified Cell Processing Center (CPC) in Japan. It is derived from the serum of carefully screened, healthy donors. Every step of the manufacturing process follows strict quality and safety standards to ensure a high-purity, clinically reliable product.
The serum is processed using our patented method, and the final product is sterile filtered to remove any potential contaminants. Comprehensive testing is conducted at each stage to ensure the safety, stability, and consistency of the final GcMAF solution.
See Tests of our GcMAF below for more detailed information.

How is Saisei Mirai’s New GcMAF Tested for Biological Activity?
Saisei Mirai’s New GcMAF is evaluated for macrophage-activating activity through a validated phagocytosis assay conducted at the University of Tokushima. In this test, mouse macrophages are exposed to opsonized sheep red blood cells—marked for ingestion by activated immune cells.
The degree of macrophage activation is assessed by measuring the number of red blood cells ingested by each macrophage, visualized as purple inclusions within otherwise clear cells. From these results, the Phagocytosis Index (PI) is calculated, providing a quantitative measure of GcMAF’s biological activity.
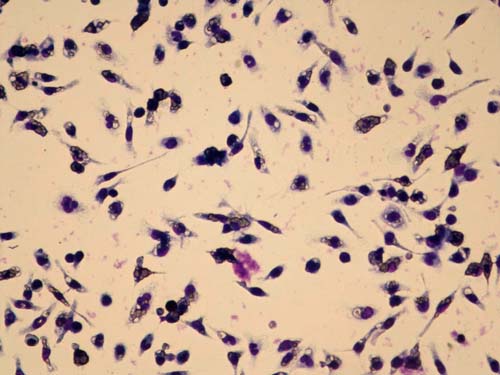
Macrophage Phagocytic Activity Induced by New GcMAF
This image shows macrophages activated by New GcMAF, actively phagocytizing opsonized red blood cells. The ingested red blood cells appear as clear structures within the purple-stained macrophages.
Photo courtesy of the University of Tokushima.
What Are Macrophages?
Macrophages—literally meaning “big eaters” in Greek—are specialized immune cells derived from monocytes, a type of white blood cell. Found throughout body tissues, they play a central role in both innate (non-specific) and adaptive (specific) immunity.
Their primary function is phagocytosis—the process of engulfing and digesting pathogens, cancer cells, and cellular debris. Macrophages can act as stationary sentinels or move throughout the body, and they also help activate other immune cells, such as lymphocytes, to mount a coordinated immune response.
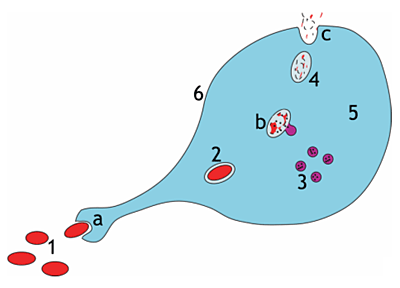
Steps of a macrophage ingesting a pathogen
a. Ingestion through phagocytosis, a phagosome is formed
b. The fusion of lysosomes with the phagosome creates a phagolysosome; the pathogen is broken down by enzymes
c. Waste material is expelled or assimilated (the latter not pictured)
Parts: 1. Pathogens, 2. Phagosome, 3. Lysosomes, 4. Waste material, 5. Cytoplasm, 6. Cell membrane
Vitamin D Binding Protein (Gc Protein)
Vitamin D binding protein (DBP), also known as Gc Protein, is a multifunctional plasma protein primarily produced in the liver, particularly in response to sunlight exposure. Its main role is to bind and transport 25-hydroxyvitamin D [25(OH)D], the circulating form of vitamin D, facilitating its storage and distribution throughout the body.
Among the different forms of DBP, the most prevalent is a non-glycosylated 656 Da protein. In addition to its role in vitamin D metabolism, DBP serves as a critical scavenger of extracellular G-actin, especially relevant in liver injury and disease. Furthermore, through specific glycosylation (GalNAc-modification), Gc Protein can be converted into a macrophage activating factor (MAF), which plays a key role in immune function.
Notably, vitamin D binding protein has minimal influence on vitamin D’s biological activity or distribution in the body, meaning elevated DBP levels generally do not pose health concerns. It is considered the primary precursor of macrophage activating factor in the human body.
Macrophage Activation Factor (MAF)
What is Macrophage Activation Factor?
Macrophage Activation Factor (MAF) refers to a group of glycoproteins that enhance the activity of macrophages, key immune cells involved in the detection and destruction of pathogens, abnormal cells, and cellular debris. MAF stimulates macrophages to become more active in immune defense, but it does not convert them into natural killer (NK) cells—these are separate immune cell types with distinct functions.
The primary precursor to MAF in the human body is Vitamin D Binding Protein (DBP), also known as Gc Protein. When Gc Protein undergoes specific glycosylation, particularly GalNAc modification, it becomes an active form of MAF. This glycosylated Gc Protein is considered one of the most effective natural activators of macrophages.
NaGaLase (Alpha-N-acetylgalactosaminidase)
NaGaLase is an enzyme produced in trace amounts in normal healthy liver cells.
What harm can NaGaLase do?
Alpha-N-acetylgalactosaminidase (alpha-NaGaLase) is produced in large amounts by cancer cells. alpha-NaGaLase deglycosylates the trisaccharide of Gc Protein at step prior to the final isoform of MAF. alpha-NaGaLase from tumors induces an immunosuppressive state that allows the cancer to spread and eventually results in death by infection.
What good can NaGaLase do?
Endo-alpha-N-acetylgalactosaminidase is produced in small amounts by probiotic bifodobacterium. NaGaLase produced in the intestine by our probiotics serves a role in breaking down mucin glycoproteins in our food.
Where else is NaGaLase found?
NaGaLase is also produced by bacteria, virus infected cells and fungi.
Normal serum levels of NaGaLase
Normal levels of NaGaLase range between 0.38 to 0.63 nmole/min/mg protein. People with cancer have NaGaLase above 2.32 nmole/min/mg protein.
Radiation therapy decreases the number of cancerous cells capable of secreting alpha-NaGaLase. Radiation also increases Gc Protein activation to principle MAF. Radiotherapy and photodynamic therapy decreases NaGaLase activity.
Target Conditions for New GcMAF Therapy
New GcMAF (Macrophage Activating Factor) therapy supports immune function and has been used as a complementary approach in the management of a wide range of conditions, including:
Cancer
Infectious diseases (including antibiotic-resistant bacteria and COVID-19)
Autism spectrum disorder (ASD)
Chronic fatigue syndrome (CFS/ME)
Atopic dermatitis
Hair loss (alopecia)
Multiple sclerosis
Pollinosis (seasonal allergic rhinitis)
Autoimmune diseases
Injury recovery support
Epilepsy
Neurodegenerative diseases
For rejuvenation and healthy longevity
In individuals with a well-functioning immune system, the body may be able to naturally fight many diseases. However, those with weakened or compromised immune responses may benefit significantly from immune modulation using New GcMAF.
Safety and Administration
Since 2011, New GcMAF has been safely administered in our clinics in Japan to hundreds of patients. The therapy is delivered by intramuscular (IM), subcutaneous (SC), or intratumoral (IT) injection, depending on the condition.
Adverse effects are rare and typically mild. Approximately 1 in 100 patients may experience a low-grade fever or temporary eczema, both of which resolve on their own.
Combining New GcMAF with Other Therapies
New GcMAF can be safely integrated with a wide range of conventional and advanced therapies. This integrative medicine approach is designed to enhance overall therapeutic effectiveness while supporting and modulating the immune system.
Compatibility with Standard Cancer Treatments
New GcMAF may be used alongside anti-cancer drugs and radiation therapy:
Chemotherapy: For best results, administer GcMAF a few days apart from chemotherapy to avoid potential suppression of macrophage activation.
Radiation therapy: Can be used concurrently with GcMAF, as radiation does not significantly interfere with its immune-stimulating effects.
In our clinical experience, combining GcMAF with palliative radiotherapy has produced promising tumor-reducing effects, especially in patients with prior chemotherapy. See our Case Reports for more information.
Integrative Combination Therapies
New GcMAF may also be used in combination with a wide range of supportive and immunomodulatory therapies, including:
Sonodynamic Therapy (SDT)
Photodynamic Therapy (PDT)
Sonophotodynamic Therapy (SPDT)
Tumor Treating Fields (TTF)
Gene Therapy (e.g., PTEN, p53)
Maitake mushroom extract
Coley Vaccine (Coley Fluid)
High-dose intravenous Vitamin C
Low-dose Naltrexone (LDN)
Alpha-lipoic acid
Whole-body or localized hyperthermia therapy
Immunotherapies and cancer vaccines, including autologous cancer vaccines
Mechanisms of Action
New GcMAF exerts its therapeutic effect by activating macrophages to target abnormal cells, including cancer. It also demonstrates anti-angiogenic properties, which may help inhibit the formation of new blood vessels that feed tumors.
Things To Avoid
Gc-MAF can be safely used with a wide variety of drugs and other treatments.
Treatment
- Treatment is by intramuscular (IM) or subcutaneous (SC) injection of GcMAF macrophage activating factor, 3-7 times per week (or as prescribed by the treating medical doctor). See Dosing Recommendations below.
- Treatment in our clinics has also been by intratumoral (IT) injection, although IM and SC injection is by far the most common means of administration.
- Good aseptic technique with ethanol is required when using the vials.
Dosing Recommendations For Second Generation GcMAF
- The dosage and frequency of second-generation GcMAF administration should be determined by the treating physician and/or the patient, based on individual health needs and response to treatment.
For further guidance, please contact us at https://saisei-mirai.or.jp/en/enq-eng/.
GcMAF Immuno Therapy
Access New GcMAF and Dietary MAF Products
Patients and healthcare providers can obtain New GcMAF therapy (injectable) developed by Saisei Mirai through our official Saisei Mirai online shop.
For oral formulations, our Dietary MAF series is available via the Saisei Pharma online shop.
High Dose GcMAF 2.5 ml multi-dose vials (1500 ng/0.5 ml):
- Each multi-dose vial contains at least 4 doses GcMAF at 0.5 ml/dose. Vials are overfilled to 2.5 ml, so up to 5 doses GcMAF may be possible for each vial.
- Shipping and handling fee is from 18,000 JPY by FedEx.
- Please keep the vials in the freezer. Only the vial you are using is kept in the fridge.
When frozen, it helps preserve activity for a very long time and prevents the settling of some solid parts in the GcMAF serum, which may start to occur with a few weeks to months of sitting in the fridge.
Stability tests indicate that 2nd Generation GcMAF is very temperature stable and retains maximum activity even after 4 weeks at room temperature and 1 week at 40 °C (104 °F). Shipping will not affect the activity of the product. Please refer to our Stability of GcMAF experiment report on our website for laboratory data.
- Please keep the vials in the freezer. Only the vial you are using is kept in the fridge.
- Please contact us for pricing.
Information
If you wish to order Second/Third Generation GcMAF Therapy made by Saisei Mirai, please visit our official Saisei Mirai online shop, or for oral MAF series products, our Saisei Pharma online shop.
- GcMAF is a natural immunotherapy product. Variation in GcMAF concentration is due to normal variation between blood samples. Just like Lymphocytes or Natural Killer cells vary in number between people and at any given time, so will the amount of Gc-MAF.
- Charges for shipping and handling apply to each order. We use FedEx with tracking information. Each package includes cooling packs to keep GcMAF at a stable temperature during shipping. Our Second Generation GcMAF is strong enough at room temperature to maintain a high level of activity for the days in transit. See more details about the Stability of GcMAF in Serum (PDF) report below for the tests done by the University of Tokushima on stability by phagocytic activity of our GcMAF after short-term and long-term storage.
- We ship Gc-MAF as a liquid in high quality multi-dose vials. After arrival the vials should be stored in the Freezer to maintain maximum activity until each injection.
Estimated Stability of New GcMAF
New GcMAF retains its biological activity for a minimum of 2 weeks at room temperature and at least 1 year under refrigerated conditions (2–8 °C).
Recent testing confirms that New GcMAF remains stable for up to 4 weeks at room temperature without any significant loss of activity.
However, for optimal preservation and long-term stability, we recommend storing vials in the freezer.
Shipping Notes for New GcMAF
We use specialized packaging with ice packs to maintain stable temperatures during transit. New GcMAF is formulated to remain biologically active at room temperature for several days, making it robust enough to withstand typical shipping durations without compromising efficacy.
All shipments contain fresh, liquid-form New GcMAF, prepared just before dispatch to ensure optimal quality. Based on stability testing, the product is expected to arrive in excellent condition with no loss of activity.
For more details, please refer to Stability Tests of New GcMAF below.
Tests of Second Generation GcMAF
Testing and Quality Assurance of New GcMAF
For full details, please visit our Tests of New GcMAF page.
New GcMAF is produced in our state-of-the-art, sterile Cell Processing Center using a patented, enhanced method that yields a product 10–20 times more concentrated than conventional first-generation GcMAF. This advanced formulation offers greater bioactivity, improved stability, and a high level of purity.
To ensure the highest standards of quality and safety, each batch undergoes rigorous testing:
Sterile filtration using a 0.22-micron medical-grade filtration system
Endotoxin testing to confirm product safety before release
Activity assays to verify macrophage activation capability
All procedures are performed under strict aseptic conditions to meet clinical-grade manufacturing standards.
Our Screening Process Includes the Following:
- TPHA test (Syphilis)
- Hepatitis B surface antigen (HBsAg)
- Hepatitis B core antibody (HBcAb)
- Hepatitis B e antigen (HBeAg)
- Hepatitis C virus (HCV) antibody
- HIV antigen and antibody
- Human T-cell lymphotropic virus (HTLV1) antibody
- Endotoxins
Activity Tests of New GcMAF:
Activity testing of New GcMAF was conducted by researchers at the University of Tokushima. The following results apply specifically to our New GcMAF (formerly known as 2nd generation GcMAF).
Stability Under Various Conditions
Room temperature (10–15 °C) for 14 days: No significant loss of activity
High temperature (40 °C) for 7 days: No significant loss of activity
Refrigerated (2–8 °C) for 1 year: Activity remains stable
Summary of Findings
These tests confirm that New GcMAF is highly temperature stable, maintaining full biological activity even under elevated temperatures for short periods. It remains effective after:
1–2 weeks at temperatures up to 40 °C, making it suitable for worldwide shipping
1 year under refrigeration, with no measurable loss in potency
For long-term storage, we recommend keeping vials in the freezer to preserve optimal stability.
Research Papers and Presentations
Please refer to our research papers and presentations page for detailed information.



